
Белоусова
Татьяна Геннадьевна
учитель химии
высшей категории
КГУ «ОШ»№ 95 г.Алматы
КГУ «ОШ» № 95
г.Алматы
УРОК ХИМИИ
В 10 КЛАССЕ
УЧИТЕЛЬ ХИМИИ БЕЛОУСОВА Т.Г.

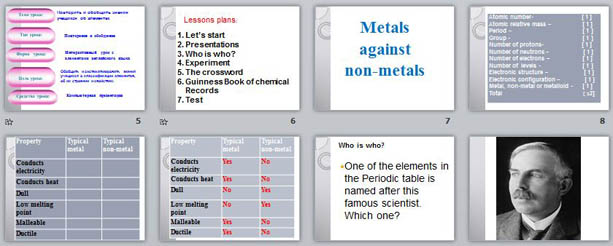
Metals
against
non-metals

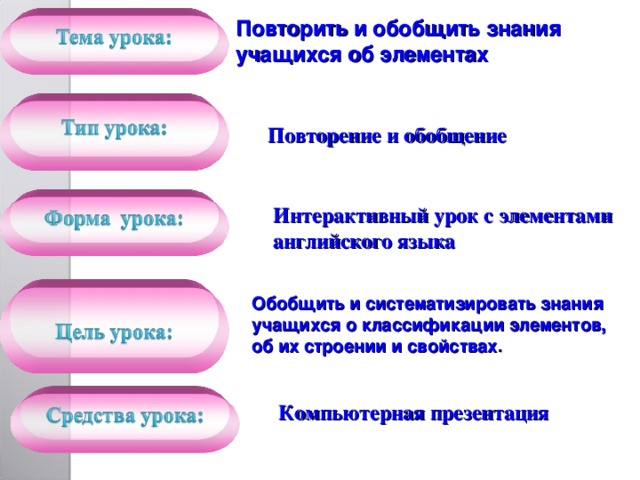
Повторить и обобщить знания учащихся об элементах
Повторение и обобщение
Интерактивный урок c элементами английского языка
Обобщить и систематизировать знания учащихся о классификации элементов, об их строении и свойствах .
Компьютерная презентация

Lessons plans :
1. Let’s start
2. Presentations
3. Who is who ?
4. Experiment
5. The crossword
6. Guinness Book of chemical
Records
7. Test

Metals
against
non-metals
![Atomic number- [ 1 ] Atomic relative mass – [ 1 ] Period – [ 1 ] Group - [ 1 ] Number of protons- [ 1 ] Number of neutrons – [ 1 ] Number of electrons – [ 1 ] Number of levels - [ 1 ] Electronic structure – [ 1 ] Electronic configuration – [ 1 ] Metal, non-metal or metalloid - [ 1 ] Total [ 1 2]](https://fsd.videouroki.net/html/2014/07/02/98683867/img7.jpg)
Atomic number- [ 1 ]
Atomic relative mass – [ 1 ]
Period – [ 1 ]
Group - [ 1 ]
Number of protons- [ 1 ]
Number of neutrons – [ 1 ]
Number of electrons – [ 1 ]
Number of levels - [ 1 ]
Electronic structure – [ 1 ]
Electronic configuration – [ 1 ]
Metal, non-metal or metalloid - [ 1 ]
Total [ 1 2]
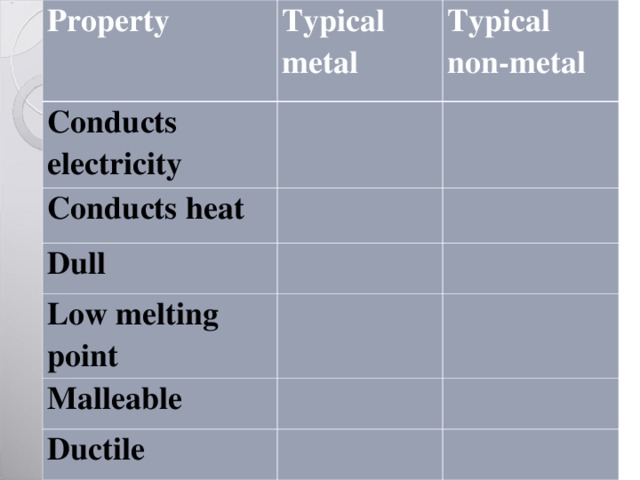
Property
Typical metal
Conducts electricity
Typical non-metal
Conducts heat
Dull
Low melting point
Malleable
Ductile
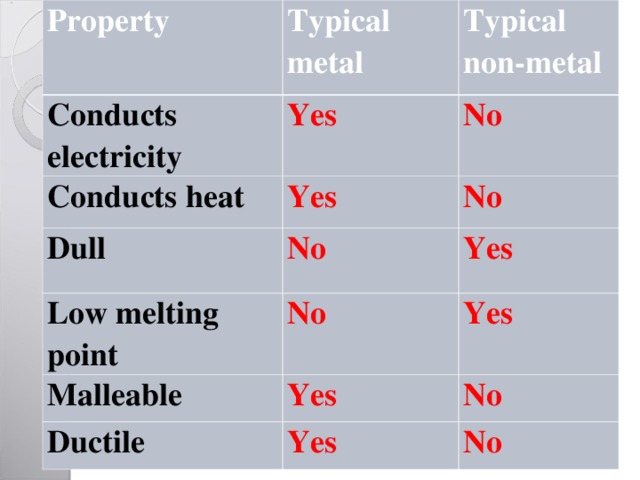
Property
Typical metal
Conducts electricity
Typical non-metal
Yes
Conducts heat
No
Yes
Dull
Low melting point
No
No
Yes
No
Malleable
Yes
Yes
Ductile
No
Yes
No

Who is who ?
- One of the elements in the Periodic table is named after this famous scientist. Which one?











Experiment
Testing the pH
of different solutions
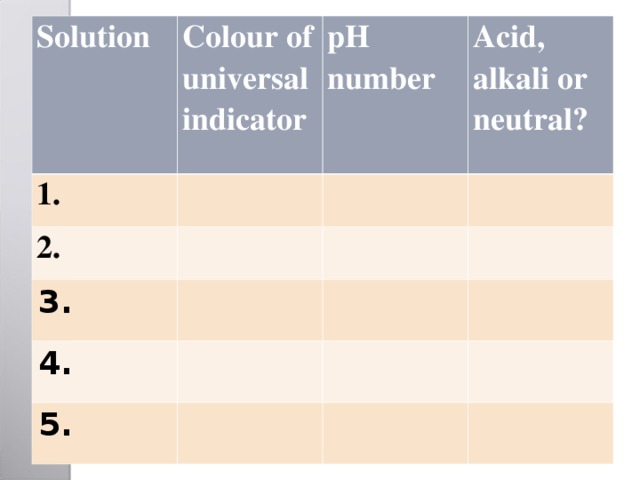
Solution
Colour of universal indicator
1.
2.
pH number
Acid, alkali or neutral?
3.
4.
5.
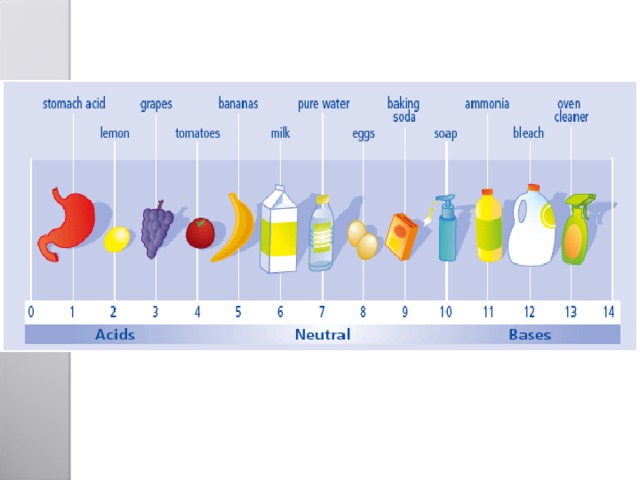
 7 neutral alkali " width="640"
7 neutral alkali " width="640"
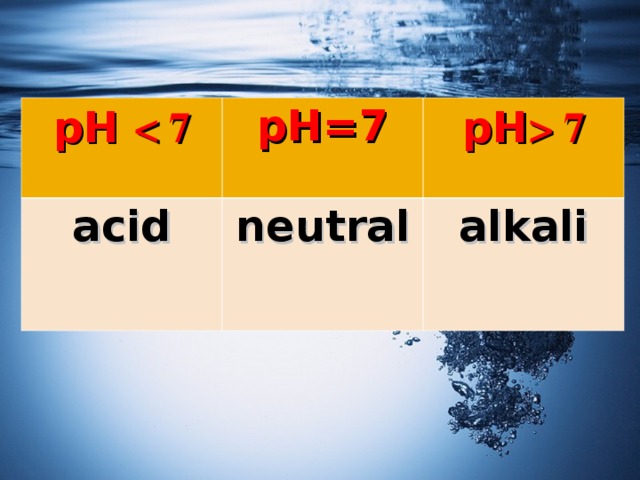
pH
pH=7
acid
pH 7
neutral
alkali

The crossword
- This element has atomic number of 6
- It has 1 proton
- This element is associated with Europe
- This element’s number is 12
- This element’s is 4 period 8 group B
- This element has atomic mass of 32
- It is associated with Titan
- This element has symbol of Ra
- This element is 39 period 5 group B
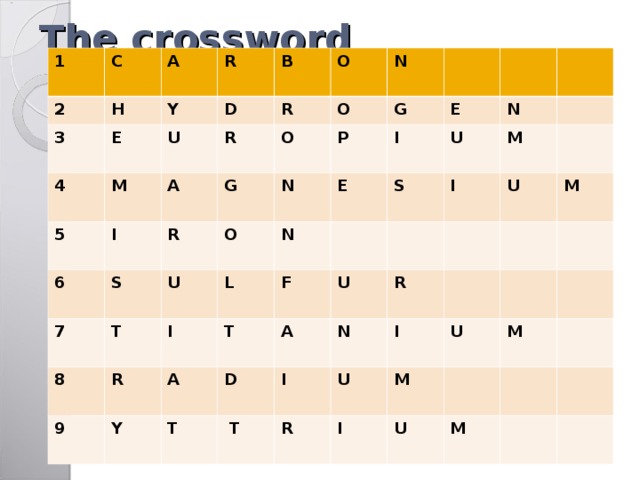
The crossword
1
C
2
3
A
H
R
4
Y
E
M
U
5
B
D
I
R
R
A
6
O
R
G
O
S
N
7
O
N
O
T
8
G
U
P
N
I
R
E
E
L
I
9
A
N
T
Y
F
U
S
A
T
M
I
D
U
U
T
I
R
N
R
M
U
I
M
U
I
M
U
M

Guinness Book of chemical Records
The most common element on the Earth is …
Oxygen

Guinness Book of chemical Records
- The strongest metal is …
- Francium

Guinness Book of chemical Records
- The strongest non-metal is …
- Fluorine

Guinness Book of chemical Records
- The most expensive element is …
- Californium

Guinness Book of chemical Records
- The lightest element is …
- Hydrogen

Guinness Book of chemical Records
- The heaviest metal is …
- Osmium

Guinness Book of chemical Records
- The best conductor of electricity is …
- Silver

Guinness Book of chemical Records
- The most wide spread metal is …
- Aluminum

Guinness Book of chemical Records
- The most of malleable metal is …
- Gold

Guinness Book of chemical Records
- The most poisonous element is …
- Fluorine
Test

TEST
1. Choose the formula of an
acidic oxide :
A ) Na 2 O
B ) N 2 O 5
C ) CaO
D) Al 2 O 3
E) Fe 2 O 3

TEST
2.Choose the formula of a base :
A ) Na 2 O
B ) KOH
C ) CaCO 3
D) Al 2 O 3
E) FeS

TEST
3. In the solution of an alkali
phenolphthalein changes its to :
A ) crimson
B ) blue
C ) red
D) black
E) white
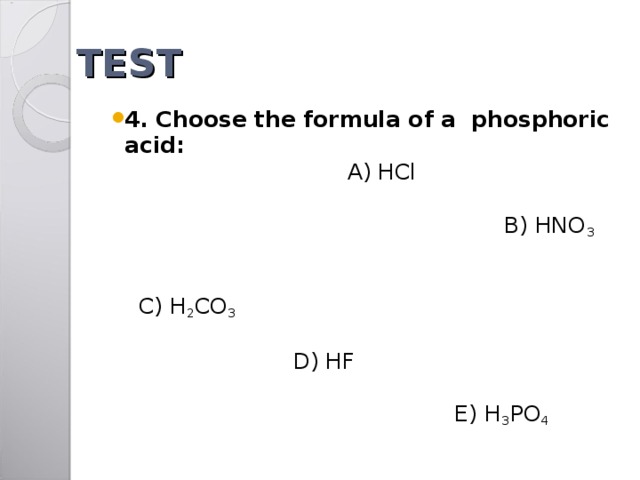
TEST
- 4. Choose the formula of a phosphoric acid: A) HCl B) HNO 3 C) H 2 CO 3 D) HF E) H 3 PO 4

TEST
- 5. Choose the formula of a magnesium nitrate:
A) MgCl 2
B) Mg(NO 3 ) 2
C) MnCO 3
D) MnF 2
E) Mn(NO 3 ) 2

TEST
- 6. Choose the formula of the substance which doesn , t belong to the logical group:
A) K B) Na C) Ca D) S E) Mg

TEST
- 7.Sodium hydroxide reacts with each of two substances:
A) HCl, CuO
B) H 2 O, Na 2 O
C) Cu(OH) 2 , SO 3
D) H 2 SO 4 , CO 2
E) MgO, HNO 3

TEST
Fe + CuSO 4 → X + Y
X and Y respectively:
A) FeO, CuS
B) FeSO 4 , CuS
C) FeO, CuSO 3
D) FeS, CuO
E) FeSO 4 , Cu
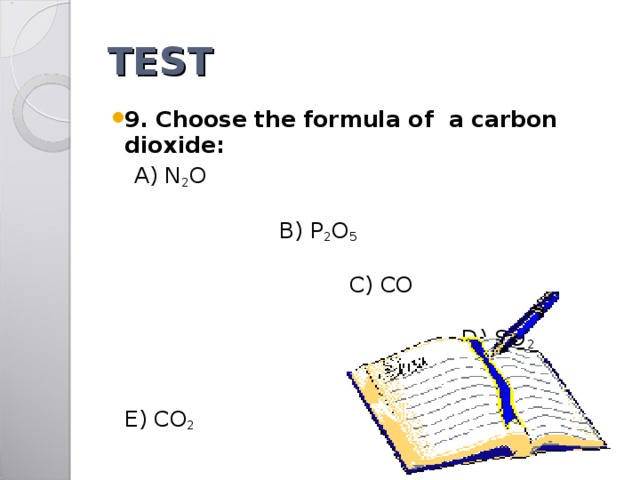
TEST
- 9. Choose the formula of a carbon dioxide:
A) N 2 O B) P 2 O 5 C) CO D) SO 2 E) CO 2
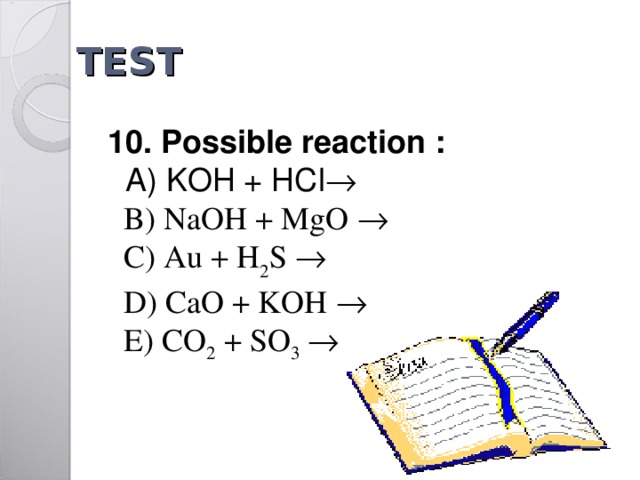
TEST
1 0 . Possible reaction :
A) KOH + HCl
B) NaOH + MgO
C) Au + H 2 S
D) CaO + KOH
E) CO 2 + SO 3


Write your opinion about the lesson :
It was interesting _____
It was important ________
It was necessary _____

Results


HOME WORK
Read book.
Write structure elements
N 30, 35

Список используемых материалов
1.Интернет-ресурсы
2. Виртуальная химическая лаборатория
3.Все опыты по неорганической химии
4.Центральные образовательные ресурсы
Ссылки на фотографии:
http://www.animelove.su/_fr/2/3487359.jpg
http://www.otkrytie.ru/photo/nepal/annapurna.jpg


 Получите свидетельство
Получите свидетельство Вход
Вход









![Atomic number- [ 1 ] Atomic relative mass – [ 1 ] Period – [ 1 ] Group - [ 1 ] Number of protons- [ 1 ] Number of neutrons – [ 1 ] Number of electrons – [ 1 ] Number of levels - [ 1 ] Electronic structure – [ 1 ] Electronic configuration – [ 1 ] Metal, non-metal or metalloid - [ 1 ] Total [ 1 2]](https://fsd.videouroki.net/html/2014/07/02/98683867/img7.jpg)
















 7 neutral alkali " width="640"
7 neutral alkali " width="640"









































 Презентация по химии на русском и английском языке по теме "Металлы против неметаллов" (3.77 MB)
Презентация по химии на русском и английском языке по теме "Металлы против неметаллов" (3.77 MB)
 0
0 3046
3046 319
319 Нравится
0
Нравится
0


